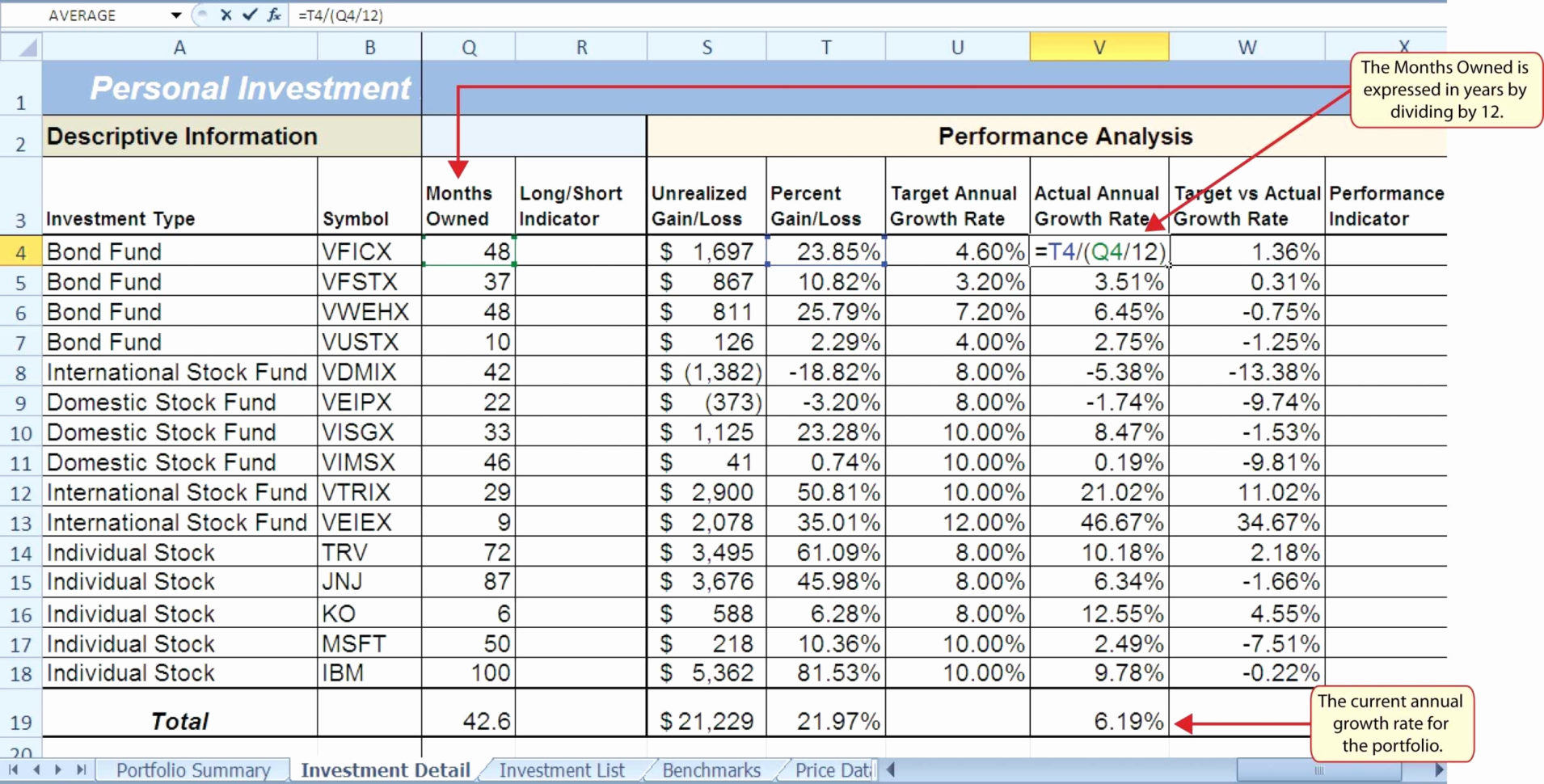
Where to Submit Your Drug Course Paperwork Easily

In today's fast-paced academic and professional world, submitting your drug course paperwork efficiently and accurately is crucial. Whether you're a student preparing for pharmacology exams or a professional working in the pharmaceutical industry, understanding where and how to submit your paperwork can significantly impact your academic journey or career progression. This post explores various avenues for submitting drug course documentation, ensuring you meet deadlines and maintain compliance with regulatory standards.
Understanding the Importance of Submission Platforms

Before diving into where you can submit your drug course paperwork, let’s appreciate why submission platforms are essential:
- Accuracy: Ensures your work is properly credited and evaluated.
- Timeliness: Keeps you on track for deadlines, preventing late submissions.
- Compliance: Helps you adhere to university or professional body requirements.
- Record Keeping: Provides a digital footprint of your academic and professional endeavors.
Where to Submit Your Drug Course Paperwork

1. University or College Platforms

If you’re a student, your primary platform for submitting drug course paperwork will be your university or college’s learning management system (LMS). Here are common platforms used by institutions:
- Moodle: Many universities utilize Moodle, allowing students to upload assignments, participate in forums, and track grades.
- Blackboard: Another widely used platform where you can submit paperwork through the assignment feature.
- Canvas: Increasingly popular in North America, Canvas offers a user-friendly interface for submitting coursework.
- Direct Email: Occasionally, professors might request submissions through email, typically as an attachment with specific naming conventions.
💡 Note: Always check your university’s guidelines for specific submission protocols; deviations might result in penalties or missing marks.
2. Professional Bodies and Continuing Education Providers

Professionals might submit paperwork to maintain certification or for continuing education credits through:
- Online Portals: Most professional organizations have digital platforms where members can upload coursework or certification requirements.
- Conferences and Seminars: Submission of coursework might be required for participating in or presenting at conferences.
- Hard Copy: Although becoming less common, some might require mailing physical documents, which needs to be planned well in advance.
3. National and International Drug Regulatory Agencies

When it comes to regulatory compliance, you might need to submit to agencies like the FDA (Food and Drug Administration), EMA (European Medicines Agency), or MHRA (Medicines and Healthcare products Regulatory Agency):
- Online Submissions: Regulatory agencies often have portals for submitting applications for new drug approvals, adverse event reporting, or periodic safety updates.
- Electronic Common Technical Document (eCTD): Used for marketing authorization submissions in the EU.
- e-Submission Gateway: Systems like this are utilized by the FDA for efficient electronic submissions.
📋 Note: Ensure your submissions comply with the specific regulatory framework, as errors can delay approvals or lead to rejection.
Best Practices for Submission

To ensure your drug course paperwork submission goes smoothly, follow these best practices:
- Understand Deadlines: Mark all deadlines on a calendar or reminder system.
- Check Formatting Requirements: Some platforms or agencies have strict formatting guidelines.
- Backup Your Work: Save copies of your work both online and offline.
- Proofread: A final check for errors can save you from resubmissions.
- Follow Naming Conventions: Adhere to any file naming rules to make it easy for reviewers to identify your work.
- Track Your Submission: Keep records of your submission dates and confirmation emails if available.
The world of drug courses and regulatory compliance requires meticulous attention to detail, especially when it comes to paperwork submission. Understanding the platforms and best practices for submission not only keeps you compliant but also enhances your reputation for professionalism and reliability. By submitting your drug course paperwork through university platforms, professional bodies, or regulatory agencies, you're not just completing a task; you're taking a significant step in your educational or career journey. Remember, the key to successful submission lies in understanding the process, being timely, and maintaining accuracy. The effort you put into ensuring your paperwork is correctly submitted will reflect positively on your academic and professional records, opening doors to further learning and opportunities in the pharmaceutical world.
How do I know where to submit my drug course paperwork?

+
Check with your institution or the organization requiring the submission. Often, there are guidelines or an LMS portal, or specific emails from the professor or administrative office.
Can I submit my paperwork via email, or does it have to be through an online portal?

+
It depends on the institution or organization’s policy. Always confirm the submission method before sending. Email submissions might be allowed but ensure you follow any naming conventions or protocol.
What should I do if the submission platform experiences technical difficulties?

+
Contact the IT department of your institution or the platform’s support team immediately. Also, keep a backup of your work in case resubmission is necessary.
How do I ensure my drug course paperwork is compliant with regulatory standards?

+
Review the guidelines provided by the regulatory agency. Often, they provide templates, checklists, and specific submission formats to ensure compliance.
Is it possible to submit drug course paperwork after the deadline?

+
Depending on the institution or regulatory body’s policy, late submissions might be penalized or not accepted. Always aim to submit on time, but if you miss a deadline, communicate with the relevant parties to see if there’s any flexibility.